Report by working group and committee [Exhibition Committee]
Report on the International Technical Exhibition of Medical Imaging 2009
The International Technical Exhibition of Medical Imaging 2009 (ITEM 2009) was held concurrently with JRC 2009 (the 68th Annual Meeting of Japan Radiology Congress, the 65th Scientific Assembly of the Japanese Society of Radiological Technology, and the 97th Scientific Assembly of the Japan Society of Medical Physics) over three days from the 17th (Fri.) to the 19th (Sun.) of April, 2009 in the Pacifico Yokohama Exhibition Hall.
ITEM is the biggest event in the activities of the JIRA Exhibition Committee. This Exhibition is sponsored by the Japan Radiology Congress (JRC) which consists of four organizations; i.e., the above-stated three academic societies and JIRA, and is operated by JIRA. All members of the Exhibition Committee share operation and management tasks to ensure the realization of a smooth operation of the exhibition.
The Exhibition has been aiming at internationalization for the past several years and has been re-named the ITEM since 2001 to invite participants also from various Asian countries. It is expected that the Exhibition will continue to be scaled up in the future.

The ITEM 2009 was held using Halls A, B, C and D of the entire Pacifico Yokohama Exhibition Hall (exhibition space was 8,780 m2 of 20,000 m2). Over the past several years, the exhibition space had not been enough, in particular in 2007 and 2008, due to an increase in the total exhibition space required from the growth in the number of participating companies. This had caused problems such as the flow of people being disrupted due to narrow passages resulting from the congested booths layout and because only small resting places had been allowed within the exhibition site. For the International Technical Exhibition of Medical Imaging 2009, Hall A was available to secure broader passages and plentiful spacious resting places. This helped solve the aforementioned problems and complaints from the participating companies. In the spacious exhibition site, 144 companies (equipment exhibitors (141) and overseas academic societies (3)) participated to demonstrate their latest pieces of equipment, related products and software to visitors.
With the main theme, “The Glorious Path of Radiation Medicine, from Now to the Future: The harmony between human and technology” ITEM 2009 was inaugurated with the opening ceremony held in front of the main entrance to the exhibition site in the exhibition halls at 9:30am on the 17th April (Fri.). In the ceremony, Mr. Endo, Chairperson of JRC from the sponsor side and Mr. Inomata, Chairperson of JIRA from the operator side delivered opening speeches. A performance
by the Yokohama City Fire Band added grace to the ceremony.
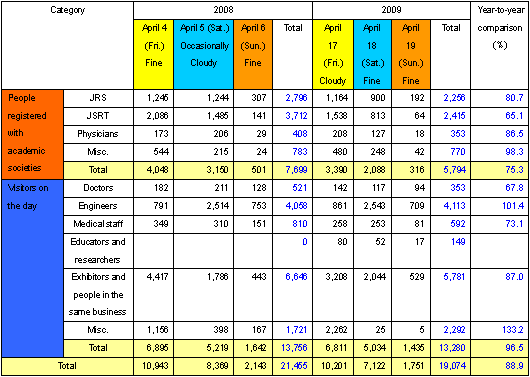
The worldwide economic downturn that started with American sub-prime loans in 2008 has also affected Japan, resulting in scale-downs or cancellations of various events and declination of participants. Under such circumstances, the International Technical Exhibition of Medical Imaging 2009 was held with almost all of the exhibition applicants participating. A substantial reduction in the number of visitors had been presumed, however, as many as 19,074 actual visitors came to the Exhibition during the exhibition period (Table 1). This represents approximately only an 11 % decrease over the previous year. The exhibition halls were so full of people that the decrease in the number of visitors seemed to have no influence on the exhibition itself. (Fig.1)

In the International Technical Exhibition of Medical Imaging 2009, a presentation section was set up in the JIRA unit as a new project (Fig. 5). This project was intended to allow the participating companies to make the most out of the ITEM 2009 by delivering a presentation utilizing JIRA's wider exhibition space. Priority was given to the companies allocated with a unit with basic fittings to give them an opportunity to perform demonstrations of their products and introduction of their technology by a narrator and company staff similarly with the participants allocated with a more spacious unit. The participants using a unit with basic fittings had not been able to give narrations or presentations due to the lack of space for inviting visitors. That was the reason why applications from those participants were given priority. As a result, 12 companies (28 topics) used the JIRA's presentation section for presentation. Since the project was the first attempt, there were some issues with respect to prior notification, set-up location for the project, size of monitors and sound volume. For next year and beyond, we will try to improve the details of the project to further respond to the participants' needs.
 |
| Fig. 1 |
Spreadsheet of the number of visitors to the International Technical Exhibition of Medical Imaging 2009
[comparison with the number of actual (newly registered) visitors in the previous year] |
 |
JIRA booth exhibition in the 61st CMEF (Spring Fair in Shenzhen city)
-Report by the working group on international exhibitions-
1. Overview of the 61st CMEF
Period: April 18 to April 21, 2009
Venue: Shenzhen Convention & Exhibition Center
Venue space: Total area 110,000 m2
Number of visitors: Approx. 57,000
Number of participating companies: Approx. 2,100 (20 countries)
The China International Medical Equipment Fair (CMEF) advocates the Asia's largest scale commercial exhibition held in China. Since 1979, it has been held twice, in spring and in autumn, every year sponsored by CAMDI (China Association for Medical Devices Industry). This time, it was the 5th exhibition of the JIRA booth in CMEF following the 60th CMEF (autumn) in Suzhou. Two members of the working group on international exhibitions participated in the 61st CMEF not only for the exhibition but also for its operation.
2. Overview of the JIRA booth
| Number of participating companies: |
4 companies exhibited actual machines/devices
and 3 companies exhibited catalogs |
| Booth space: |
54 m2 |
| Location of the booth: |
Japan Village in the International Exhibition Zone |
| |
|
 |
| Fig. 1 Scene of exhibition in the JIRA booth |
The JIRA booth was set up at the Japan Village in the International Exhibition Zone to exhibit panels introducing JIRA, international activities and ITEM 2010 as well as the relevant devices and equipment applied by the JIRA member companies.
In addition, a JIRA introduction leaflet in Chinese, JIRA members list, participating companies' catalogs and commemoratives were distributed to the visitors. The visitors were requested to fill out a questionnaire asking several questions such as in what way the visitors were interested in the JIRA activities. The questions were the same as those in the previous CMEF 2008 Autumn Fair. The questionnaire was adopted with the intention of making JIRA's exhibition activities more effective in the future.
With respect to the question about the responders' occupation, medical devices sales workers such as local agencies occupied more than a half of the significant answers (2,154 as shown in the chart). The percentage of doctors and hospital workers increased in comparison with the previous questionnaire result. In addition, the variation in the visitors' occupation slightly widened.
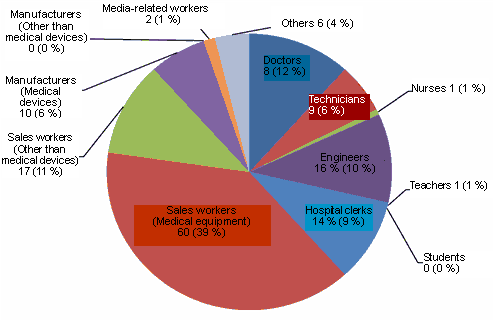 |
| Fig. 3 The occupations of visitors to the JIRA booth |
3. Overview of the international exhibition
The CMEF Spring Fair is larger-in-scale and more international when compared with the Autumn Fair. One of its features is the International Exhibition Zone which was described as a large scale international pavilion.
In the Spring Fair, Hungary, Norway, Finland and Malaysia additionally made an exhibition with their national flag shown.
One of the new attempts in the Spring Fair was the set-up of a pavilion for companies which specifically specialized in IVD.
The U.S.A., Korea, U.K., Germany, Spain, France, Japan, Hungary and Singapore were indicated on banners in the venue. In addition, Malaysia, Finland and Scotland collectively set up a pavilion and Ireland and Canada did the same.
In the Japan pavilion in the Spring Fair, seven companies respectively had a booth in addition to the JIRA booth. Four of the seven companies participated in CMEF for the first time.
4.Exhibition tour by JETRO staff
In the Spring Fair, two JETRO staff (from JETRO Hong Kong) were invited to actually experience CMEF in terms of its huge scale and the governmental support to the participating companies.
We guided them for more than about four hours through the participating countries' booths. In each booth, we received favorable comments about the existence of JIRA from governmental officers of the respective participating countries. This was a really significant response to JIRA since such favorable comments helped us effectively appeal our international exhibition business to the JETRO staff.
Through the tour, we also acquired information on the framework of the pavilions of each of the participating countries. In the past, participating companies seemed to have been supported mainly by their countries. Recently, however, an increasing number of participating companies are supported by their cities and states/provinces.
As a conclusion, all the participating companies except for the Japanese ones were more or less supported at the national or local governmental level. We've heard that the IVD pavilion from France, in particular, was substantially supported. The Japanese participants in the Japan pavilion asked the JETRO staff to positively support them.
In the meeting held after the tour, the JETRO staff told us that JETRO had supported exhibitions in the past but not nowadays. They added that JETRO currently focuses on the food industry. However, it was a great progress that the JETRO staff actually visited CMEF. In addition, we received a positive response from them. We will therefore continue to work on JETRO aggressively for their support.
5.Issues to be addressed
The inquiries received in the JIRA booth has not only increased in number but has also become more specific with each exhibition. It seems numerous Chinese companies and agencies keenly wish to deal with Japanese companies.
It is a known fact that Japanese companies have to conform to the medical device regulations of overseas countries before advancing into foreign markets including the Chinese market. The procedure of applying for Chinese medical device approval involves a particularly large amount of paper work. We have received many requests for support from our working groups on the relevant devices.
Incidentally, we heard that administrative departments of foreign countries respond to such requests in this manner. Again, JETRO or other public authorities support may be essential in regard to this issue.
The Spring Fair of CMEF was fruitful to JIRA. We could show JIRA’s updated activity with aiming to provide more opportunities for overseas activities of our member companies. Our activity could also contain the possibility of future governmental support. We will examine the information obtained in the 61st CMEF and make the most out of it in our international exhibition activities in the future.
Report about the participation
in the DICOM Standards Committee (Kyoto)
The DICOM Standards Committee (DSC) manages and maintains the DICOM Standards. Its meeting was held on the 21st of April, 2009 in Kyoto City. Since the meeting was held in Japan, the JIRA Bureau supported the Committee with respect to the venue selection and provision of information for participants.
1. Status of DSC
DSC is the highest decision-making body that sets and maintains the DICOM Standards as well as totally supervises and gives direction to activities of individual working groups (WG). The DSC Standards have already covered a wide variety of products. And yet, the existing standards are revised from time to time to additionally define new techniques and information in accordance with the progress of medical devices and medical technology and to improve the vast number of text pages by correcting spelling errors or by modifying wording to more appropriate ones.
Relatively substantial changes and additions are proposed as a "Supplement," and corrections of spelling errors and re-wording of texts are proposed as a "Correction" respectively by the relevant working group (WG). In the case of it being necessary to publish a major supplement, the working group in charge submits a "New Work Item Proposal (NWIP)" to DSC. DSC discusses the proposal to decide whether the actual work for the publication of the supplement is to be started. Since a supplement is published to notify a relatively large change, the working group in charge develops the final draft to be submitted to DSC through repeated discussions by means of teleconferences (T-con) and mailing lists (MLs). Since the number of corrections has substantially increased these days, simple corrections of words and phrases are often processed collectively. At present, 144 supplements and 980 corrections have been numbered.
2. 1st DSC meeting 2009 in Kyoto
The first DSC meeting in 2009 was held on the 21st of April, 2009 in Kyoto. The schedule and venue of a DSC meeting are determined habitually about a year in advance. The meeting in Kyoto had been determined during the DSC meeting held in April 2008 in China. The DSC meetings held in 2008 and 2009 and those to be held toward the end of 2009 are listed below:
2008: April in China, June in Germany and December in the U.S.A.
2009: April in Japan, June in Greece and December in the U.S.A.
As listed above, the meetings are held in Asia, Europe and America in turn.
If the DICOM standards are converted into XML-based ones, retrieval and reference of a target standard would become far easier. Expectations of the people concerned are therefore very high. However, it must be a tremendous workload to convert the aforementioned huge volume of the standards into XFL-based ones and reformat the associated diagrams and tables. The progress of this work was also reported in the Kyoto meeting. It seems that a longer period would be needed until the work can be completed. In addition, NWIPs were made by two working groups and approved by the Committee. These two proposals will be put on the ballot in the near future.
With respect to the restrictions on the translation and reference of the DICOM standards which came to be an issue from about a year ago, MITA (Medical Imaging and Technology Alliance) bureau provided a summary report on the points to be considered. With the attempt to translate the entire DICOM standards, JIRA has been engaged in translation and checking year after year. In the meeting, JIRA received a comment from the Committee that publishing Japanese versions of the entire body of the standards would cause no problem as long as they would be published only for the reference purpose in consideration of the continual changes in the DICOM standards.
Then, the DICOM-related organizations in various countries made reports on their activities. Only a limited number of participants attended the Kyoto meeting. Only the standards promotion bodies in Europe (COCIR), Taiwan (MISAT) and Japan (JIRA) and SCO(Standards-development-organization Charter Organization), HITSP/B(Healthcare Information Technology Standards Panel/Board), ACR(American College of Radiology), IHE-NA(Integrating Healthcare Enterprise-North America) in the U.S.A. made reports. No reports were provided from Canada, China, India and Korea and HL7 and ISO/TC215.
The working groups reported the progress of their activities made after the DSC meeting in RSNA last year. The meeting was adjourned after confirmation of the schedule of DSC meetings in the future.
3.JIRA report materials
The summary of reports made by JIRA is as follows:
- Introduction of DICOM Workshop held in Japan mainly by IHE-J
We made a relatively detailed explanation of the DICOM Workshop since the IHE-NA desired to use the Workshop as a model for the DICOM/IHE TF (Technical Framework) promotion activities.
- Reporting that JJ1017 had been revised to V3.1 (Version 3.1) with the range of radiotherapy expanded and comments on the new version were being solicited from the public.
We suggested that a correction to the relevant DICOM standard would have to be proposed once the V3.1 was finalized since JJ1017 V3.0 had already been registered as a reference standard for the DICOM standards.
- Information on the progress of translation of the DICOM standards
We reported that 16 sections of the DICOM standards had already been translated into Japanese and published; nine of them were translations of the 2001 version and seven of them were translations of the 2008 version, and translation of the remaining DICOM standards would be continuously carried out.
 |
| Photo Full view of the DSC meeting in Kyoto |
As the host country, we are very happy to have successfully completed the DSC meeting in Kyoto.
We will continue not only to provide support to the setting and expansion of the DICOM standards in active manner but also to further contribute to the DSC through proposal of new standards from Japan.
ISO committee
ISO-TC215 Meeting at Edinburgh
Typical ISOs that JIRA attends are ISO/TC210 (Quality management and corresponding general aspects for medical devices) and ISO/TC215 (Health informatics). As part of JIRA's activities, the Security Committee of JIRA is a member of WG4 (Working Group 4: Security) of ISO/TC215 and the DICOM Committee is a member of WG2 (Working Group 2: Data interchange). This report describes the activities, in particular, of the Security Committee, a member of WG4.
1. The course of the ISO committee
An ISO/TC215 meeting for general experts is usually inaugurated with an opening plenary session, followed by the respective working groups meetings on their work and is closed with a closing plenary session. The opening plenary session is often one-hour long and is arranged in the first day morning of the term of the committee meeting. Since this time slot is used for greetings as described above, participants exchange courtesies. The major time is consumed for the working session of the ISO/TC215 meeting. Those meetings consume approximately more than 60 % of the total committee meeting time to discuss individual documents for standardization. In the closing plenary session, the working groups respectively make reports on the discussions they have made and the committee member countries provide their views on the work of the working groups.
 |
Photo: Scene of the meeting on the work |
2. Meeting at Edinburgh
2.1 Opening Plenary session
Dr. Kwak, who was the Chairman of ISO/TC215 delivered an opening address to inaugurate the meeting, followed by delivery of an address by Chairman of CEN (European Committee for Standardization) and an organizer of the committee meeting. Then, the Secretary of ISO/TC215 did a roll call to check the member countries. From this meeting onwards, Singapore participated as an observer.
2.2 Working groups meetings on their work
As one of the changes made by the ISO/TC315 committee, WG5 (Health cards) was dissolved. Its remaining work was assigned to WG4. We've heard that the reason was the substantial reduction in the entire work volume of WG5. As a result, the work volume was too small to be undertaken by one dedicated working group.
The major items of discussion on standardization were as follows:
- DTS 29321 Application of clinical risk management to the manufacture of health software
- DTR 29322 Guidance on the management of risk to ensure patient safety of health software system in deployment and use
Those standards have been outstanding issues concerning the risk management of health software. The U.K., the proposing country of those standards, had withdrawn them from ISO and CEN and had submitted it to JWG7, which was a joint working group between IEC/SC62A (Common matters of electrical equipment for medical use) and ISO/TC215 to try to harmonize them with IEC 80001, the standard on risk management of medical devices undertaken by JWG7. This harmonization agrees with JIRA's opinion from the first place.
The standard discussed next was ISO/CD 27789 (Audit trail for EHR). Mr. Nishida, the chairperson of JIRA Security Committee made a proposal for improvement together with Mr. Okada from JAHIS. This proposal is a formal investigation item until the next meeting.
Two new proposals were also discussed. One was "Classification of data purpose for processing of person health information" which was based on the issuance of certification in terms of security of health software. Certification and approval are delicate matters in every country. Japan has to keep an eye on it. Another was "Security aspects of EHR record migration (TR)" on data migration from the legacy system to the latest one. This proposed standard was intended to lay down some rules or arrangements to be conformed upon data migration from a security standpoint.
2.3 Closing Plenary Session
The outcome of the meetings of the working groups held in the past several days were reported. In the closing plenary session, the DTR 11636 (the Draft Technical Report 11636 on a dynamic on-demand virtual private network for health information infrastructure) which was submitted by Japan, was passed by a majority in the vote.
The committee meeting at Edinburgh brought to light once again the fact that standardization efforts made by the European countries were far more advanced than that by other countries. Many standards proposed by European countries are finalized in reflection of the environment and culture of Europe partly because they are discussed by CEN. There has already been an established cycle where the European countries set standards, then they propose them as international standards such as ISO, and once those standards are established as international standards, the European countries press the rest of the world for compliance to the established international standards against the backdrop of the gigantic commercial sphere of EU. It is assumed that European countries are entering the stage where they can make a choice of which standards are essential for them.
The next ISO/TC215 will be held in the coming October in Durham (North Carolina), U.S. A. |

