Results
(1) Creation of rules for software for medical use
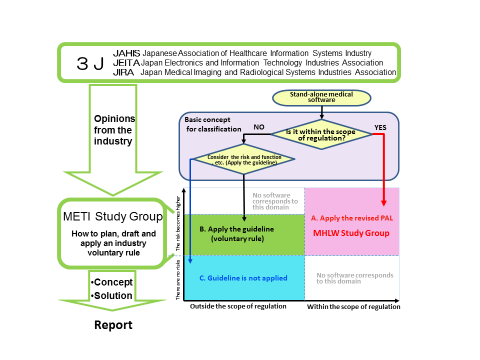
The 3J members (JAHIS, JEITA and JIRA) joined "the Medical Software Study Group” that was set up by the Ministry of Economy, Trade and Industry (METI). They led the argument in the Study Group. As a result, they pointed out the risk caused by the so-called health software that is outside of the scope of the regulation. They also proposed a possible solution.
Moreover, the 3J members joined "the Medical Equipment Development Guideline -- Medical Software Working Group" that was newly set up in METI. They discussed how to plan and apply an industry voluntary rule for health software that is outside the scope of the regulation. They proposed establishment of the (tentatively called) Health Software Promotion Council.
(Industry Strategy Planning Office)
(2) Safety measures for MRI
An accident occurs when a metallic object is carried into the MR examination room. We issued a consistent warning not to carry a metallic object into the MR examination room. However,the number of accidents, did not decrease. We produced a checklist to warn users about MR accidents. Additionally, we produced a DVD for the same purpose in cooperation with "The Safe MRI Examination Group” and it is now available for sale.
(Regulation and Safety Division)
(3) International standardization
The basic policy is "the standardization based on international harmonization." JIRA cooperated with the “National Committee for IEC/SC 62B, SC62C " in prompt deliberation for IEC standards draft and actively attended at international conferences. As a result, our opinion was reflected on IEC standards through the related Subcommittees (SCs). However, medical software is deliberated by SC62A. Software is incorporated into JIRA products and stand-alone software is also developed. Therefore, we needed a scheme which can reflect our opinion on IEC standards. Under the Standardization Division, we established a new “Software Standards Working Group” that cooperates with JEITA in order to reflect our opinion on IEC standards.
Recently, people have highly expect technical progress of low-invasive therapy and diagnosis, such as radiation therapy. Japan excels in the field of radiation therapy. We submitted a new proposal for the standard for radiation therapy, which was approved as a New Work Item Proposal (NWIP). From now on, this item will be supported by the related Subcommittee (SC) of the Standardization Committee.
(Standardization Division, National Committee for IEC SC62B/C)
We joined ISO/TC215 WG4 Security dealing with standardization of international information security and the Security Privacy Committee (SPC) composed of the industrial associations of Japan, the U.S. and Europe. We collected and shared global security information and sent the information to member companies.
(Medical Imaging System Division)
(4) Creation of JIS
We revised JIS Z4910 Diagnostic X-ray imaging equipment−Characteristics of general purpose and mammographic anti-scatter grids and submitted its draft to the Japanese Standards Association (JSA) in February 2014.
(JIRA Committee for Standardization) |

